SOAP
AND DETERGENT MANUFACTURE
Md. Kaysar Hasan
Saurav
Roll: 08168; 14th Batch,
IER, DU.
Date of submission: 07 March, 2010.
CONTENT
Topic Page
1. SOAP AND DETERGENT MANUFACTURE 01
-Introduction
-The Chemistry of Soap
and Detergent Function
2. THE SOAP MANUFACTURING PROCESS 02-09
- The
Colgate-Palmolive Process
03
- The Lever Rexona
Process
06
- Laundry or 'hard'
soap manufacture 08
- Toilet soap
manufacture
09
3. THE DETERGENT MANUFACTURING PROCESS 09-11
- Detergent powder
manufacture
09
- Liquid detergent
manufacture
11
4. ANCILLIARY PROCESSES
12
- Glycerine
recovery
5. ENVIRONMENTAL IMPLICATIONS
13-14
- Synthetic
detergent biodegradability 13
- Detergent powder
14
6. ROLE OF THE LABORATORY
14-15
- Batch process soap 15
- Detergent powder
15
- Liquid detergent
15
7. REFERRENCES
15
SOAP AND
DETERGENT MANUFACTURE
Introduction
Soap is integral to our society today, and we
find it hard to imagine a time when people were kept sweet-smelling by the
action of perfume rather than soap. However, the current widespread use of soap
is only a very recent occurrence, despite the fact that it has been made for
more than 2500 years. The first recorded manufacture of soap was in 600BC, when
Pliny the Elder described its manufacture by the Phoenicians from goats tallow
and ash, and it was known among the British Celts and throughout the Roman Empire . However, these people used their soap medicinally,
and it was not until the second century AD that it was used for cleaning, and
not until the nineteenth century that it began to be commonly used in the Western
world.
Early
this century the first synthetic detergents were manufactured, and these have
now taken the place of soap for many applications. Their manufacture is covered
briefly in the second part of this article.
The Chemistry of Soap and Detergent Function
All
soaps and detergents contain a surfactant1 as their active ingredient This is
an ionic species consisting of a long, linear, non-polar ’tail’ with a cationic
or anionic ’head’ and a counter ion. The tail is water insoluble and the head
is water soluble - a difference in solubility which has two important implications.
Firstly, this makes the surfactant molecule a wetting agent: the tails migrate
to align themselves with the solid: water interface, lowering the surface
tension at that point so that it penetrates the fabric better. Secondly, it
allows the oily dirt particles to form an emulsion with the water: the tails of
many surfactant molecules surround an oily dirt particle, forming a micelle
with a drop of oil in the centre and the ionic heads of the surfactant
molecules pointing outwards and hence keeping the micelle in the polar
solution.
THE
SOAP MANUFACTURING PROCESS
The essence of soap production is the
saponification reaction:
This reaction is exothermic, and
progresses quickly and efficiently at around 125oC inside an autoclave type
reactor.
The most common fats and oils used are tallow (beef or mutton/beef
blend), coconut oil, and palm kernel oil. Different oils produce soaps of
varying hardness, odor and lathering, so the ratios of the oils used are
closely monitored to produce a blend with the most desirable characteristics
for the most reasonable cost.
However,
pure soap is hard and easily oxidized, so various additives are added to
correct this and to make a more aesthetically pleasing product. The first such
"additive" is glycerin, which is produced in the saponification
reaction. Glycerin makes the soap smoother and softer than pure soap. However,
it is also much more valuable than soap itself, so only a minimum of glycerin
is left in the soap and the remainder is extracted, purified and sold.
The glycerin is extracted from the soap with
lye2 - a brine solution that is added to the soap at the saponification stage.
Wet soap is soluble in weak brine, but separates out as the electrolyte
concentration increases. Glycerin, on the other hand, is highly soluble in
brine. Wet soap thus has quite a low electrolyte concentration and is about 30%
water (which makes it easily pump able at 70oC). To remove the glycerin, more electrolytes
are added, causing the wet soap to separate into two layers: crude soap
and a brine/glycerin mixture known as spent lye, neutral lye or sweet waters.
The soap still contains some salt, which itself functions as an additive,
altering the viscosity and color of the soap.
Once
the spent lye has been removed the soap is dried, chipped, mixed with other
additives such as perfumes and preservatives and then plodded (squeezed
together), formed into tablets and packaged for sale.
There are two different soap-making
processes used in New
Zealand
The Colgate-Palmolive Process
This is a continuous process (Figure
1) which uses a plant built by Binacchi & Co. The z process is best
understood in terms of two streams: soap flowing in the order given below against
a counter-current of lye.
Step 1 – Saponification
The raw materials are continually fed
into a reactor in fixed proportions. Assuming a production rate of 1000 kg wet
soap per hour and a 80:20 tallow: coconut oil mix, the raw materials would be
fed in at the following rates:
Coconut oil 525.9 kg hr-1
Tallow 131.5 kg hr-1
50% NaOH solution 101 kg hr-1*
*Although this is not the formula
quantity, it gives a general indication to the process condition. The actual
amount is affected by the caustic concentration in half - spent lye.
These ingredients alone would give a
low water, high glycerin soap. Soap needs to be about 30% water to be easily pumpable,
and even then needs to be held at around 70oC, so excess lye is added to
hydrate the soap and dissolve out some of the glycerin. The lye added is known
as "half spent lye" and is the lye discharged from the washing column.
This lye already contains some glycerin, but it is further enriched by that
formed in the saponification reaction.
Step 2 - Lye separation
The wet soap is pumped to a
"static separator" - a settling vessel which does not use any mechanical
action. The soap / lye mix is pumped into the tank where it separates out on
the basis of weight. The spent lye settles to the bottom from where it is piped
off to the glycerin recovery unit, while the soap rises to the top and is piped
away for further processing.
Step 3 - Soap washing
The soap still contains most of its glycerin
at this stage, and this is removed with fresh lye in a washing column. The
column has rings fixed on its inside surface. The soap solution is added near
the bottom of the column and the lye near the top. As the lye flows down the column
through the centre, a series of rotating disks keeps the soap / lye mixture
agitated between the rings. This creates enough turbulence to ensure good
mixing between the two solutions.
The rate of glycerin production is
calculated and the rate at which fresh lye is added to the washing column then
set such that the spent lye is 25 - 35 % glycerin. Glycerin is almost infinitely
soluble in brine, but at greater than 35% glycerin the lye no longer
efficiently removes glycerin from the soap.
The soap is allowed to overflow from
the top of the column and the lye ("half spent lye") is pumped away
from the bottom at a controlled rate and added to the reactor.
Step 4 - Lye separation
The lye is added at the top of the
washing column, and the soap removed from the column as overflow. As the lye is
added near the overflow pipe the washed soap is about 20% fresh lye, giving the
soap unacceptably high water and caustic levels. Separating off the lye lowers
the electrolyte levels to acceptable limits.
The soap and lye are separated in a
centrifuge, leaving a soap which is 0.5% NaCl and 0.3% NaOH, and about 31% water.
The lye removed is used as fresh lye.
Step 5 – Neutralization
Although the caustic levels are quite
low, they are still unacceptably high for toilet and laundry soap. The NaOH is
removed by reaction with a weak acid such as coconut oil (which contains
significant levels of free fatty acids), coconut oil fatty acids, citric acid
or phosphoric acid, with the choice of acid being made largely on economic
grounds.
Some preservative is also added at this
stage.
Step 6 – Drying
Finally,
the water levels must be reduced down to about 12%. This is done by heating the
soap to about 125oC under pressure (to prevent the water from boiling off while
the soap is still in the pipes) and then spraying it into an evacuated chamber
at 40 mm Hg (5.3 kPa). The latent heat of evaporation lost as the water boils
off reduces the soap temperature down to 45oC, at which temperature it solidifies
onto the chamber walls.
The soap chips are scraped off the walls
and "plodded" (i.e. squeezed together) by screws known as
"plodder worms" to form soap noodles. The soap is now known as base
or neat soap chip, and can be converted into a variety of different soaps in
the finishing stages.
The moisture evaporated off the wet soap
is transported to a barometric condenser, which recompenses the vapor without
the system losing vacuum. The moisture can contain soap dust (.Fines.) which is
removed by cyclones and returned by augers to the spray chamber, while the
water is recycled.
Base soap can also be made by a batch
process such as that used by Lever Rexona.
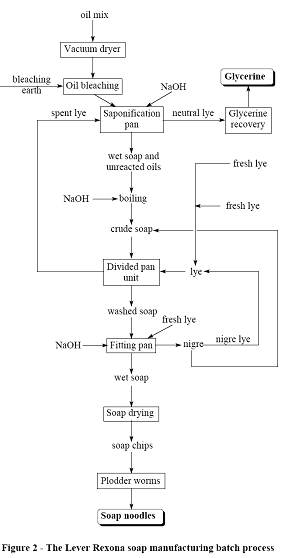
The Lever Rexona Process
This
process is summarized in Figure 2.
Step 1 - Oil preparation
The oils used most commonly are, as in
the Colgate-Palmolive process, tallow and coconut oil. These are blended
together and dried in a vacuum chamber. Once the oils are dry, bleaching earth
is sucked by the vacuum into the chamber to remove any colored impurities. The
spent earth is land filled and the oils stored ready for saponification.
Step 2 – Saponification
The mixture of bleached oils is mixed
with spent lye from the washing stage and a caustic soda solution. The mix is
heated and then left to settle into two layers. The neutral lye (which is now rich
in glycerin) is pumped off and the mixture of soap and unreacted oils which has
risen to the top is left in the pan. More caustic liquor is added to this and
the mix reheated to saponify the remaining free oils.
Step 3 – Washing
The crude soap is then pumped to a
divided pan unit (DPU) where it is washed by a countercurrent of lye. This lye
is a mixture of fresh brine solution and nigre lye. The washed soap comes out
the far end of the DPU and is sent to the fitting pans, while the lye comes out
the near end and is pumped back into one of the saponification pans.
Step 4 – Fitting
Here the remaining unwanted glycerin is
removed from the soap by reboiling with water, NaCl and a small amount of NaOH
solution. The electrolyte concentration in the water is such that the soap and
water to separate out into two layers. The top layer is ’neat’ wet soap, which
is pumped off to be dried. The bottom layer is known as the ’nigre’ layer, and
consists of a solution of soap, glycerin and NaCl. This is left in the pan,
reboiled with further salt and left to stand, forming a soap crust over a lower
layer of nigre lye (salt and glycerin). This soap is left in the pan and is
mixed with the next intake of washed soap, while the nigre lye is pumped back to
the DPUs to wash the next batch of crude soap.
Step 5 – Drying
Moisture is flashed off under vacuum in
the same manner as was described above for the Colgate-Palmolive process.
Laundry or 'hard' soap manufacture
The base soap is mixed with color and
preservatives and milled. Perfume is then added and the mixture plodded then
extruded into a continuous bar. This, in turn, is cut into billets and stamped
out into tablets ready for packaging.
Toilet soap manufacture
Toilet soap has less water and more
fatty material (fatty acids and soap) than laundry soap. For this reason base
soap intended for toilet soap manufacture usually has extra fatty acids added
with the preservatives before it is vacuum dried. These ensure that there is no
unreacted caustic left in the soap by the time it reaches the consumer, and
also make the soap softer. Perfume, dye and pacifier are then added to the
dried soap and the mixture milled to ensure even mixing. It is then plodded and
extruded out as a continuous bar, cut into billets and stamped ready for
packaging and sale.
THE
DETERGENT MANUFACTURING PROCESS
Detergents use a synthetic surfactant
in place of the metal fatty acid salts used in soaps. They are made both in powder
and liquid form, and sold as laundry powders, hard surface cleansers, dish
washing liquids, fabric conditioners etc. Most detergents have soap in their mixture
of ingredients, but it usually functions more as a foam depressant than as a surfactant.
Detergent powder manufacture
Step 1 - Slurry making
The solid and liquid raw ingredients (Table
2) are dropped into a large tank known as a slurry mixer. As the
ingredients are added the mixture heats up as a result of two exothermic reactions:
the hydration of sodium tri-polyphosphate and the reaction between caustic soda
and linear alkylbenzenesulphonic acid. The mixture is then further heated to
85oC and stirred until it forms homogeneous slurry.
Step 2 - Spray drying
The slurry is deaerated in a vacuum
chamber and then separated by an atomizer into finely divided droplets. These
are sprayed into a column of air at 425oC, where they dry instantaneously. The
resultant powder is known as ’base powder’, and its exact treatment from this
point on depends on the product being made.
Step 3 - Post dosing
Other ingredients are now added, and
the air blown through the mixture in a fluidiser to mix them into a homogeneous
powder. Typical ingredients are listed in Table 3.
Liquid detergent manufacture
Step 1 - Soap premix
manufacture
Liquid detergent contains soap as well
as synthetic surfactants. This is usually made first as a premix, and then
other ingredients are blended into it. This step simply consists of neutralizing
fatty acids (rather than fats themselves) with either caustic soda (NaOH) or
potassium hydroxide.
Step 2 - Ingredient
mixing
All ingredients except enzymes are
added and mixed at high temperature. The ingredients used in liquid detergent
manufacture are typically sodium tri-polyphosphate, caustic soda, sulphonic
acid, perfume and water. The functions of these ingredients have been covered above.
Step 3 - Enzyme addition
The mixture is cooled and milled, and
the enzymes added in powder form.
ANCILLIARY PROCESSES
Glycerin recovery
As has already been stated, glycerin is
more valuable than the soap itself, and so as much of it as possible is
extracted from the soap. This is done in a three step process.
Step 1 - Soap removal
The spent lye contains a small quantity
of dissolved soap which must be removed before the evaporation process. This is
done by treating the spent lye with ferrous chloride. However, if any hydroxide
ions remain the ferrous ions react with them instead, so these are first removed
with hydrochloric acid:
HCl + NaOH →
NaCl + H2O
The ferrous chloride is then added.
This reacts with the soap to form an insoluble ferrous soap:
FeCl2 +
2RCOONa → 2NaCl + (RCOO) 2Fe
This precipitate is filtered out and
then any excess ferrous chloride removed with caustic:
2NaOH + FeCl2
→ Fe (OH)2 (s) + 2NaCl
This is filtered out, leaving a
soap-free lye solution.
Step 2 - Salt removal
Water is removed from the lye in a
vacuum evaporator, causing the salt to crystallize out as the solution becomes
supersaturated. This is removed in a centrifuge, dissolved in hot water and
stored for use as fresh lye. When the glycerin content of the solution reaches
80 - 85%, it is pumped to the crude settling tank where more salt separates
out.
Step 3 - Glycerin
purification
A small amount of caustic soda is added
to the crude glycerin and the solution then distilled under vacuum in a heated
still. Two fractions are taken off - one of pure glycerin and water. The glycerin
thus extracted is bleached with carbon black then transferred to drums for
sale, while the water/glycerin fraction is mixed with the incoming spent lye and
repeats the treatment cycle.
ENVIRONMENTAL IMPLICATIONS
Soap is designed as a product to be used
once then flushed down the drain, so as expected the environmental implications
of its manufacture are not nearly as great as many other chemical processes.
There are two main areas of concern: the safe transport and containment of the raw
materials and the minimization of losses during manufacture.
The three main components of soap by both cost
and volume are oils, caustic and perfumes. Oils and perfume are immiscible in
water and if spilled create havoc, although the oils do solidify at room
temperature. Transport of these products is by trained carriers, and the systems
for pumping from the truck to storage tanks are carefully designed. Perfumes
are bought in lined steel drums which are quite robust, and flammable perfumes
are not used in soaps.
All storage tanks are surrounded by bunds to
catch the contents of a tank should it rupture or a valve fail. When the
storage system is designed, all the safety features (such as access to tank and
valves) are designed in, as well as procedures to deal with the product should
it end up in the bounded area.
Within the plant, all the process areas are
also bounded, and the trade waste from there piped to an interception tank
before draining to the council’s trade waste system. The contents of the
interception tank are continuously monitored for acidity or alkalinity, and are
designed to settle out excess solids or light phase chemicals. If a spill is
detected in the plant itself, a portion of the interception tank can be
isolated off and the effects of the spill neutralized before the waste is
dumped.
In most cases, however, potential problems are
identified and stopped before they happen. Often an off-spec product can be
reprocessed and blended rather than dumped, and even washout water can be
reprocessed to minimize the discharges from the plant.
Finally,
the manufacturing process itself is closely monitored to ensure any losses are
kept to a minimum. Continuous measurements of key properties such as
electrolyte levels and moisture both ensure that the final product is being
made to spec, and ensures the manufacturing process is working as it was
designed to. Hence the losses in the plant will indirectly be minimized because
the process itself is being monitored.
Synthetic detergent biodegradability
There has recently been a strong move away
from the environmentally hazardous biologically stable detergents used in the
past to biodegradable ones. The sulphonic acid and nonionic detergents used in New Zealand New Zealand
Detergent powder
Detergent
powder manufacture has some specific environmental issues associated with it
that are not present in other areas of the industry. These are dust control and
volatile organic emissions. Dust present during delivery and transfer of bulk
powdered detergent (and powdered raw materials) is a potential problem. Dry and
wet cyclones are used to filter out most of the dust, and all emissions are
monitored. If the dust level in these does exceed acceptable limits,
appropriate remedial action is taken. Dust levels in emissions must be kept below
50 mg m-3.
The spray drying tower also releases volatile
organics. These emissions are minimized by having tight specifications on what
can be added as primary detergent active material. Any potentially hazardous
material is added with the secondary actives after the tower so that it is not
heated. Spot checks are done on the total hydrocarbon content of the exhaust
gases using a flame ionization detector.
ROLE OF THE LABORATORY
The
laboratory monitors the formulation and specification of products from raw
material to finished goods. Much soap is formulated locally, and the laboratory
tests a range of formulations for stability and manufacturing practicality. The
trial formulations are aged in a warm oven to simulate a couple of years of
shelf life, and then checked for perfume loss or alteration, base odor, color
stability and any general rancidity. Formulations are also constantly checked
for cost effectiveness, and soaps are frequently reformulated for cost and supplier
considerations.
When a new formula has been agreed the
laboratory will lay down the specifications that the finished soap and its
intermediary stages must meet. These could be color, odor, moisture or
electrolyte concentrations, or the concentrations of impurities or additives.
These specifications are also constantly being revised as the production
equipment is improved, or consumer demands change.
The laboratory lays down all the
specifications for raw materials to be purchased against. These specifications
become the basis for the supplier to quote against. The materials are constantly
tested against these specifications, either on a shipment basis or supplier’s
batch size. In some cases the manufacturing plant is inspected and approved,
and if the supplier can validate their process then the need for many routine
or expensive tests can be reduced or eliminated.
In most cases quality testing is performed at
the process, by the process operators. The laboratory hold samples of every
batch of finished goods for twelve months, so that if there are any consumer
complaints, an original sample can be tested against the defect sample to determine
the cause of the complaint.
Tests carried out on some particular
products are listed below.
Batch process soap
The incoming tallow and coconut oil are tested
for colour (after bleaching) and free fatty acid content. The neat liquid soap
is tested for free alkali, salt content and glycerol content, while the soap
chips are tested to moisture and fatty acid content.
Detergent powder
On-line tests are continuously carried
out on density and moisture. The laboratory also tests for the concentrations
of active detergent, sodium tri-polyphosphate, moisture, soda ash, enzymes and
bleach, and monitors physical properties such as dynamic flow rate, compressibility,
particle size, color and perfume.
Liquid detergent
The product is typically tested for
viscosity, pH, cationic detergent (fabric conditioner)
content,
enzyme content, conductivity (a measure of detergent stability), color and
perfume.
REFERRENCES
•
The Enclyclopædia Britannica (15th ed.); Encyclopædia Britannica,
Inc.; 1979
• Selinger, Ben; Chemistry in the
Marketplace (3rd ed.); Harcourt Brace Jovanovich;
1986






very good knowledge.
ReplyDelete